
CE marking for masks, face coverings and face shields used for protection against Covid-19
- Connexions
- 2020-07-03
- 3021
There are a lot of people who either do not properly understand the certification requirements for medical devices and personal protective equipment (PPE), or who are wilfully ignoring them. The current Covid-19 crisis is providing cover for all kinds of confusion and the regulatory bodies are overwhelmed with other priorities. Hopefully this article will help readers to understand the true situation.
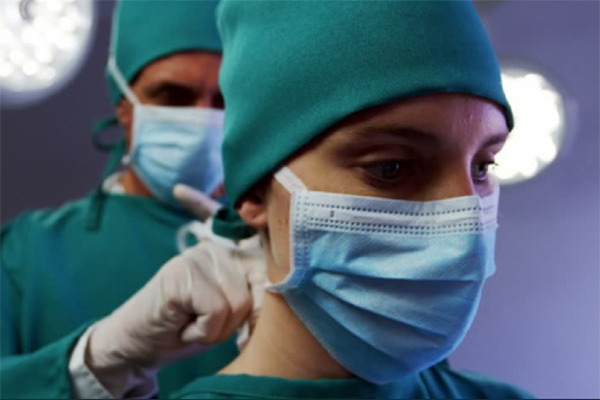
A surgical face mask, which is worn by clinical staff and is intended to stop them from transmitting an infection to their patients, is a class 1 medical device. There is a standard for them (EN 14683) which is harmonised under the Medical Devices Directive and for practical purposes following that standard is the only sensible way to conform to the CE marking requirements.
Protective face shields (also known as 'visors'), which provide protection to the medical staff and others who wear them, are PPE and under the categorisation system for that Regulation (which is completely different to the classification system used for medical devices) they are the highest category of PPE (Cat III) because they are intended to provide protection against harmful biological agents. Again, there is a standard (EN 166), harmonised under the PPE Regulation, and following this is by far the easiest way to show that you have met the requirements for CE marking.
Respiratory masks which are intended to filter particles to prevent them being breathed in by the wearer (often known as "FFP2", FFP3" or "KN95" masks) are also Category III PPE. They must comply with the performance requirements of EN 149 if they are to be CE marked.
Category III PPE must be independently type tested and manufactured under a quality system which is approved by a Notified Body. Class 1 medical devices can be CE marked by their manufacturer without any Notified Body intervention.
Legally, there is no such thing as a "EN 166 category 1 face shield” - the category is defined by the Regulation, not by the standard – and while a manufacturer may describe their product as a ‘medical face shield’ that does not change the fact that it must be certified as PPE and not as a medical device.
EN 14683 recognises that surgical face masks may also provide some protection for the wearer but it does not address this in a way which provides the level of protection required for PPE. If protection for the wearer is required then a mask complying with the PPE Regulation must be used.
CE marking a face shield as a medical device is theoretically possible but there is no standard for this and in the absence of a widely agreed performance benchmark the rules for CE marking medical devices, which require the manufacturer to have clear evidence that the device provides a medical benefit in response to one or more specific illness or disability, actually make it likely that it will cost more and take longer than the Notified Body procedure for PPE.
In response to the Covid-19 crisis, and based on recommendations from the European Commission, the UK government has introduced two fast-track procedures which allow certain types of PPE to be sold without the CE mark.
Firstly, if you are selling PPE direct to the government then the Office of Product Safety and Standards (OPSS) will assess the product and say whether or not it meets the needs of the public healthcare sector. No Notified Body is involved in this procedure, and the product cannot be CE marked. Only products sold to central government procurement are permitted to use this route - if you are selling direct to end users (including hospitals and care homes) then you must follow the second route.
The second route is that the UK Government has introduced a temporary derogation from the requirement to CE mark PPE for manufacturers whose products will be useful in responding to the Covid-19 crisis. This derogation does not remove the requirement for CE marking completely, it merely allows a manufacturer to ship their product to a customer before the CE marking process is fully complete, so long as the manufacturer has engaged a Notified Body and the Notified Body has done some basic checks on the product. Current indications are that these checks take about a week to complete. Note that the CE mark cannot be applied to the product until the full Notified Body testing and audit has been completed.
There are also similar fast track procedures in place for some medical devices (principally ventilators) but the assessment is done by the Department for Health and Social Care (DHSC) and the Medicines and Healthcare Products Regulatory Authority (MHRA) instead of OPSS. However, for class 1 devices such as surgical masks and drapes it is likely to be quicker for the manufacturer to simply follow the correct procedure for CE marking by self certification.
In addition to the two fast track procedures offered by the Government described above, there is a third procedure for PPE which has been agreed by the PPE Notified Bodies and the European Commission. This consists of a simplified set of tests which have been selected specifically for Covid-19 related protection products. Products which pass the tests will be issued with a certificate which is only valid for a year (instead of the normal 5 years for a PPE type approval) and there is no requirement for any quality control certification.
Finally, there is a class of mask (referred to as a ‘face covering’) which does not require CE marking at all. These are simple products for which the manufacturer makes no medical benefit claims or any claims of protection for the wearer. Such face coverings consist of a simple textile rectangle covering the nose and mouth and held in place by elastic loops behind the ears, or laces tied behind the wearer's head. They help to prevent the spread of disease by limiting the effects of coughing and sneezing, but they do not provide enough filtering to stop the wearer from breathing in the virus and therefore they are not classed as PPE. The OPSS have now published a brief guide on these which is linked below. We will also be publishing more guidance on these face coverings in the next few days. Please contact us if you have any questions in the meanwhile.
-
 2022-04-26Are air purifiers environmentally friendly ?
2022-04-26Are air purifiers environmentally friendly ? -
 2022-04-26The importance of wearing a mask correctly
2022-04-26The importance of wearing a mask correctly -
 2022-04-27Connexions Air H13 True HEPA Filters
2022-04-27Connexions Air H13 True HEPA Filters -
 2022-04-29What is the use of anion function of air purifier?
2022-04-29What is the use of anion function of air purifier? -
 2022-05-08Standardize the wearing of masks, children should not be missed!
2022-05-08Standardize the wearing of masks, children should not be missed! -
 2022-05-16Hazy days, air purifiers are useful?
2022-05-16Hazy days, air purifiers are useful? -
 2022-05-16Attention everyone! Don't buy fake FFP2 masks! How do we identify?
2022-05-16Attention everyone! Don't buy fake FFP2 masks! How do we identify? -
 2022-05-17Pay attention to secondary pollution when using air purifiers
2022-05-17Pay attention to secondary pollution when using air purifiers -
 2022-05-17TOP5 pollutants that the purifier can purify
2022-05-17TOP5 pollutants that the purifier can purify
-
 2020-06-02Why do Face Masks Matter With This Coronavirus
2020-06-02Why do Face Masks Matter With This Coronavirus -
 2020-06-02How to Wear Mask
2020-06-02How to Wear Mask -
 2020-06-02Three Principles of Choice of Masks
2020-06-02Three Principles of Choice of Masks -
 2020-06-022020 Situation of Mask Market
2020-06-022020 Situation of Mask Market -
 2020-06-17What other preventative measures can you take to protect yourself from airborne substances?
2020-06-17What other preventative measures can you take to protect yourself from airborne substances? -
 2020-06-08The Advantage of Disposable Face Masks
2020-06-08The Advantage of Disposable Face Masks -
 2020-06-093 Ply Disposable Face Mask & Soft & Comfortable Ear Loop
2020-06-093 Ply Disposable Face Mask & Soft & Comfortable Ear Loop -
 2020-06-17What are the regulations for surgical face masks?
2020-06-17What are the regulations for surgical face masks? -
 2020-06-09Do I need to wear a face mask if I am quarantined?
2020-06-09Do I need to wear a face mask if I am quarantined?
CONTACT US

Connexions Technology (Dongguan) Ltd.
We are always providing our customers with reliable products and considerate services.
If you would like to keep touch with us directly, please go to contact us
